how to draw so2|SO2 (Sulfur Dioxide) Lewis Structure : Pilipinas We show two methods to find correct Lewis Structure of SO2. One uses math, the other "puzzle pieces" to give the three correct structure. There is also a video and a study .
Sentence Checker. Free online spell . Portuguese (Portugal), Romanian, Russian, Slovak, Slovenian, Spanish, Swedish, Tagalog, Tamil, Ukrainian. . When you see the text «No mistakes were found» instead of the number of errors, it means that the text is checked and contains no errors. If «Check text to get stats» is displayed instead .
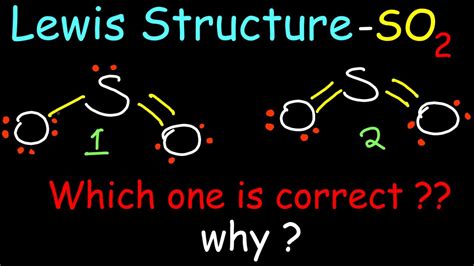
how to draw so2,A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth. This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and formal charges.To draw the SO2 Lewis structure, follow these simple steps: 1. Determine the total valence electrons. Start by counting the valence electrons of each atom in the molecule. In SO2, sulfur is in Group 6, so it has 6 .
In SO2 lewis structure, there are two double bonds between sulfur atom and oxygen atoms. Sulfur dioxide lewis structure is drawn step by step using VESPR rules. S and O atoms have sp2 hybridization.
Explanation: 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ( S ). 2. Draw a skeleton structure in which .We show two methods to find correct Lewis Structure of SO2. One uses math, the other "puzzle pieces" to give the three correct structure. There is also a video and a study . Method 1: Step method to draw the Lewis structure of SO 2. In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to get the answer. Here is a .
SO2 Lewis Structure. Before directly jumping into the lewis structure of SO2, let’s have a quick discussion regarding the importance of lewis structure and the steps to draw it. Lewis structure is the distribution . Draw the Lewis structure for SO2 – Homework.Study.com SO2 Lewis Structure in 6 Steps (With Images) – Pediabay SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry, and Bond Angles – Geometry of MoleculesTo draw the SO2 Lewis structure, follow these simple steps: 1. Determine the total valence electrons. Start by counting the valence electrons of each atom in the molecule. In SO2, sulfur is in Group 6, so it has 6 valence .
In order to calculate the formal charges for SO2 we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding elec.

What is the structure of SO 2?I have seen two different ways the Lewis Structure is written: The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. So I would assume that the one with two double bonds is the correct structure.SO2 (Sulfur Dioxide) Lewis StructureWhat is the structure of SO 2?I have seen two different ways the Lewis Structure is written: The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. So I would assume that the one with two double bonds is the correct structure. SO2 Lewis Structure Setup Step-1: To draw the SO2 Lewis structure, we have to find out the SO2 valence electrons first.We express valence electrons as dots in lewis dot structure. To get the valence electrons of sulfur,we need to look at the electronic configuration of sulfur. S(16)=1s²2s²2p⁶ 3s² 3p⁴ 3d
Draw the Lewis electron structure of the molecule or polyatomic ion. Determine the electron group arrangement around the central atom that minimizes repulsions. Assign an AX m E n designation; then identify the LP–LP, LP–BP, or BP–BP interactions and predict deviations from ideal bond angles. Describe the molecular geometry.

Step 2) Attach the atoms to each other using single bonds (“draw the skeleton structure”). Step 3) Add electrons to the outer atoms, to complete their octets. Each outer atom needs three electron pairs. Step 4) Count the electrons in each structure. Each of these structures has 24 electrons.how to draw so2 To determine the number of lone pairs and bonding pairs of electrons for SO2 we first need to draw as valid Lewis Structure. Once we have a Lewis Structure f. Sulphur dioxide gas is prepared in laboratory by heating copper turnings with conc. H 2 SO 4.. Procedure: Copper turnings are taken in a round bottom flask fitted with thistle funnel and delivery tube as shown in figure. When conc. H 2 SO 4 is poured through thistle funnel and mixture is heated sulphur dioxide gas is evolved, which is . Sometimes one Lewis Structure is not Enough . Some molecules or ions cannot be adequately described by a single Lewis structure. For example, drawing one Lewis structure for ozone (O 3) gives us a misleading picture of the actual bonding in the molecule.If we draw a Lewis structure for O 3 (ozone), we get this:. This structure .
The formal charges being zero in the above table indicate that the double-bonded SO 2 arrangement is completely stable. This is a theoretical structure obtained using formal charges- this is the structure . How to draw lewis structure for SO2? Sulfur dioxide lewis structure of SO2 , first we require the total valence electrons of both sulfur and oxygen. There are 6 valence electrons of sulfur and oxygen. This bonded with single bond . There are three resonance structures SO2 (Sulfur dioxide). We start with a valid Lewis structure and then follow these general rules. Note that SO2 is a bit. Sulfur dioxide, or SO_2, has two resonance structures which contribute equally to the overall hybrid structure of the molecule. However, a third Lewis structure can be drawn for SO_2 which is more stable in theory, but doesn't quite match experimental data. Let's draw the first two Lewis structures for SO_2. The total number of valence .
Steps of drawing SO2 lewis structure Step 1: Find the total valence electrons in SO2 molecule. In order to find the total valence electrons in SO2 (sulfur dioxide) molecule, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost .Question: Draw the Lewis structure for the sulfur dioxide (SO2) molecule. Be sure to include alli resonance structures that satisfy the octet rule Il resonance structures that satisfy the octet rule. Add atom symbol , I ←→ 2Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula S O 2.It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches.It is released naturally by volcanic activity and is produced as a by-product of copper extraction and .
In this video, we are going to figure out the shape of sulfur dioxide molecule, meaning, vsepr geoemetry for SO2. We will start by looking at the lewis stru.
how to draw so2|SO2 (Sulfur Dioxide) Lewis Structure
PH0 · What is the lewis structure for SO
PH1 · Sulfur dioxide (SO2) Lewis Structure, Hybridization
PH2 · SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagr
PH3 · SO2 Lewis Structure, Hybridization, Molecular
PH4 · SO2 Lewis Structure
PH5 · SO2 (Sulfur Dioxide) Lewis Structure
PH6 · Lewis structure of SO2 [with video and free study guide]
PH7 · Lewis structure of SO2 [with video and free study
PH8 · Lewis Structure of SO2 (sulfur dioxide)
PH9 · Lewis Structure of SO2 (With 6 Simple Steps to Draw!)